Friday, 28 August 2015
Thursday, 20 August 2015
IS MATTER AROUND US PURE
CLASS IX QUESTION ANSWERS
Q1: Which separation techniques will apply for the separation of the following?
CLASS IX QUESTION ANSWERS
Q1: Which separation techniques will apply for the separation of the following?
- Sodium chloride from its solution in water.
- Ammonium Chloride from a mixture containing Sodium Chloride and Ammonium Chloride.
- Small pieces of metal in the engine oil of a car.
- Different pigments from an extract of flower petals.
- Butter from curd.
- Oil from water.
- Tea leaves from tea.
- Iron pins from sand.
- Wheat grains from husk.
- Fine mud particles suspended in water.
- Q2How would you confirm that a colourless liquid given to you is pure water?
- Every liquid has a characteristic boiling point at 1 atmospheric pressure. If the given colourless liquid boils exactly at 373 K at 1 atmospheric pressure, then it is pure water. If the boiling point is different then the water is contaminated.
- Q.3*State the difference between simple distillation & fractional distillation.
- Fractional distillation:
1.Fractional distillation is the process of separation used mainly to separate liquid mixtures based on difference in boiling points.when the difference in boiling points of liquid components is less than 25⁰C. - Fractionating column is used in this method
- Ex: Separation of natural gas, kerosene oil…etc from petroleum done by using fractional distillation.
Distillation:
Simple distillation also used to seperate the liquid mixturesoror a mixture of solid in a liquid, when the difference in boiling points of liquid components is greater than 25⁰C. - Fractionating column is not used in the process.
- eg. Mixture of acetone and water, obtaining pure water from sea water
- Q. 9. In the formation of sodium chloride from its constituent elements, do the properties of its elements change. Explain.Ans: Sodium is a very reactive metal that reacts exothermically with water. If we were to ingest a pinch of sodium, it would burn our intestines. Chlorine is a greenish yellow gas with acharacteristic irritating odour and pungent taste. When sodium and chlorine combine to form sodium chloride, the properties of the elements are totally changed. Sodium chloride is a white substance totally safe to be ingested and is used to add flavour to our food.
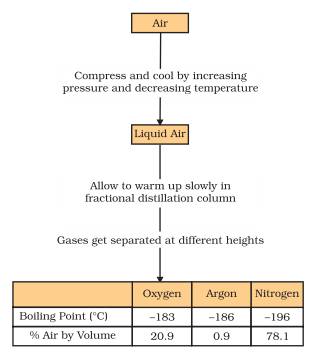
- FLOW DIAGRAM TO SEPARATE COMPONENTS OF A LIQUID
Tuesday, 4 August 2015
CLASS-9 MATTER IN OUR SURROUNDINGS
WORKSHEET
WORKSHEET
1. A gas jar containing air is inverted over another jar containing NO2 gas which is brown in colour and heavier than air.After some time brown colour is seen in the inverted glass jar too. Identify the phenomenon associated with the observation.
2.A spoonful of sugar is added to a beaker containing 500 ml water of water and stirred for a while. State any two observation that you will make.Account for your observations
.
3.In which of the following substances the inter molecular forces are the (a) weakest (b) strongest
Water, Alcohol,Carbon- di- oxide, sodium chloride
4.Give reasons-
(a) Gas fills the vessel completely in which it is kept.
(b) A gas exerts pressure n the walls of the container.
(c) Gases have high compressibility and show diffusion.
(c) Gases have high compressibility and show diffusion.
5. How would you confirm that colourless liquid given to you is pure water?
6.Explain one example of diffusion of solid into another solid.
7.Name two gases which dissolve in water by diffusion.What is importance of this process in nature
.
8.In a beam of sunlight entering a room , we can sometimes see dust particles moving in a haphazard way in the air. Why do they move?
9.Honey is more viscous than water. Why?
10.Bromine and air takes 15 minutes to diffuse but bromine diffuses in vaccum very rapidly. Why is this so?
, ************
Subscribe to:
Posts (Atom)








