Tuesday 20 November 2018
Monday 15 October 2018
class 9 holiday homework
(refer the blog's of your respective teachers)
Maths - worksheet in the blog
science- worksheet in the blog
Hindi- Blog
Social science- Group activity on Food security
Geography- Choose any one topic from the following and write about it.( Pictures ,Map ,Causes Prevention, effects)
Tsunami
Drought
Land slides
English-
1.Article on the topic (200 words)
Sedentary lifestyles and unhealthy eating habits leads to obesity
2Story writing (150- 250 words)
.ENDING. WITH... HE TOUCHED HIS GURU'S FEET AND ASKED FOR AN APOLOGY
BEGINNING WITH...AS I OPENED THE WINDOW i SAW...
3LETTER-
You recently upgraded the band of your net.However the speed is quite low and it is difficult to load websites.You have complained to customer sevices, but they are extremely unhelpful.Write a letter to General Manager World Tel internet Service provider complaining about the service
(refer the blog's of your respective teachers)
Maths - worksheet in the blog
science- worksheet in the blog
Hindi- Blog
Social science- Group activity on Food security
Geography- Choose any one topic from the following and write about it.( Pictures ,Map ,Causes Prevention, effects)
Tsunami
Drought
Land slides
English-
1.Article on the topic (200 words)
Sedentary lifestyles and unhealthy eating habits leads to obesity
2Story writing (150- 250 words)
.ENDING. WITH... HE TOUCHED HIS GURU'S FEET AND ASKED FOR AN APOLOGY
BEGINNING WITH...AS I OPENED THE WINDOW i SAW...
3LETTER-
You recently upgraded the band of your net.However the speed is quite low and it is difficult to load websites.You have complained to customer sevices, but they are extremely unhelpful.Write a letter to General Manager World Tel internet Service provider complaining about the service
Wednesday 10 October 2018
CLASS-9 HOLIDAY H W
LESSON-3 ATOMS AND MOLECULES(A-4 SIZE PAPER)
LESSON-3 ATOMS AND MOLECULES(A-4 SIZE PAPER)
|
1
|
What is an atom?
|
|
|
2
|
How many atoms are present in one molecule of ozone?
|
|
|
3
|
Define the law of constant proportion.
|
|
|
4
|
What is the ratio between masses of (a) hydrogen and
oxygen in H2O (b) nitrogen and hydrogen in NH3? [Atomic
mass of H = 1 u, O = 16 u, N = 14 u]
|
|
|
5
|
What is the ratio between masses of carbon and oxygen in
CO2?
[Atomic mass of C = 12 u, O = 16 u] |
|
|
6
|
As per the law of definite proportions carbon and oxygen
combine in a ratio of 3 : 8. Compute the mass of oxygen gas that would be
required to react completely with 6 g carbon.
|
|
|
7
|
Name the anion and cation which constitute the molecule of
magnesium oxide.
|
|
|
8
|
Name the international organisation which approves the
name given to the elements.
|
|
|
9
|
The oxide of aluminium has a chemical formula Al2O3.
State the valency of Al.
|
|
|
10
|
Write the names of the following compounds:
(a) Al2(SO4)3 (b) NH4OH |
|
|
11
|
What is the formula of ammonium chloride?
|
|
|
12
|
(a) Define atomic mass unit.
(b) Hydrogen and oxygen combine in the ratio 1: 8 by mass to form water. What mass of oxygen gas would be required to react completely with 4 g of hydrogen gas? |
|
|
13
|
(a) Give one word for the following:
(i) Positively charged ion (ii) A group of atoms carrying a charge. (b) Mention any two important rules for writing a chemical formula. |
|
|
14
|
(a) How would you differentiate between a molecule of an
element and a molecule of a compound? Write one example of each type.
(b) Write the chemical formula of baking soda. |
|
|
15
|
Define atomicity. Give an example of each of monoatomic,
diatomic, tetra-atomic and polyatomic molecules.
|
|
|
16
|
Classify the following compounds as diatomic, triatomic
and polyatomic molecules:
HCl, H2, H2O and NH3. |
|
|
17
|
How will you prove experimentally the law of conservation
of mass?
|
|
|
18
|
(a) When 5 g of calcium is burnt in 2 g of oxygen then 7 g
of calcium oxide is produced. What mass of calcium oxide will be produced
when 5 g of calcium is burnt in 20 g of oxygen. Which law of chemical
combination will govern your answer? State the law.
(b) Write the chemical formula of calcium oxide. |
|
|
19
|
(i) Write the full form of IUPAC.
(ii) Hydrogen and oxygen combine in the ratio of 1 : 8 by mass to form water. What mass of oxygen gas would be required to react completely with 3 g of hydrogen gas? |
|
|
20
|
Write the formulae of
(a) Magnesium hydroxide (b) Hydrogen sulphide (c) Potassium chloride (d) Calcium oxide (e) Barium chloride (f) Sodium carbonate |
|
|
21
|
An element ‘X’ forms an oxide with formula X2O3
(a) State the valency of X. (b) Write the formula of (i) chloride of X, (ii) sulphate of X. |
|
|
22
|
Using criss-cross method, write the chemical formulae of
copper chloride, calcium sulphate and sodium phosphate.
|
|
|
23
|
(a) What are polyatomic ions?
(b) Write the formulae and names of the compounds formed by combination of (i) Fe3+ and SO42– (ii) NH4+ and CO32– |
|
|
24
|
Write the formulae of:
(a) Sodium chloride (b) Aluminium oxide (c) Ammonium sulphate |
|
|
25
|
(a) Define polyatomic ion.
(b) Write the name of the compound (NH4)2SO4 and mention the ions present in it. |
|
Thursday 20 September 2018
REVISION WORKSHEET-CLASS 8
FORCE AND PRESSURE
FORCE AND PRESSURE
The woman wearing sandals with flat soles will feel more comfortable while walking on the sandy beach. The flat soles have larger area compared to the sandals with pointed heels. Since the two women are of the same weight, they will apply same force on the ground. Therefore, the pressure exerted by the pointed heels will be more compared to that with sandals having flat soles. As a result the pointed heel sandals will sink more in the sand than the flat sole sandals. Hence, walking with flat sole sandals will be more comfortable.
Tuesday 18 September 2018
chemical properties of
ethanoic acid



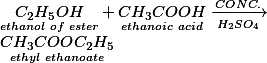
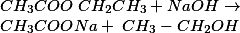
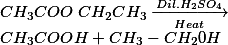
ethanoic acid
Ethanoic Acid ( /Acetic Acid :
- 5-8% solution of acetic acid in water is called vinegar.
- Pure acetic acid is called glacial acetic acid
| REACTS WITH | PRODUCTS | |
| 1. | SODIUM Na | SODIUM ETHANOATE AND HYDROGEN |
| 2. | SODIUM CARBONATE | SODIUM ETHANOATE, CARBON
DIOXIDE AND WATER
|
| 3. | SODIUM BICARBONATE | SODIUM ETHANOATE, CARBON
DIOXIDE AND WATER
|
| 4. | ETHANOL (IN PRESENCE OF CONC. SULPHURIC ACID) | ESTER AND WATER |
| 5. | NAOH | SODIUM ETHANOATE AND WATER |



esterification
Carboxylic acids react with alcohols in presence of few drops of concentrated sulphuric acid as catalyst and form sweet smelling compounds called ester.
Hydrolysis On heating with an acid or a base the ester forms back the original alcohol and carboxylic acid.
- Alkaline hydrolysis of ester is also called saponification.
Soaps and Detergents
- Soap is sodium and potassium salt of carboxylic acids with long chain.
- Soaps are effective with soft water only and ineffective with hard water.
- Detergents are ammonium or sulphonate salts of carboxylic acids with long chain. They are effective with both soft as well as hard water.
An ionic part (hydrophilic) and a long hydrocarbon chain (hydrophobic)part constitutes the soap molecule.
 Long chain of carboxylic acid, hydrophobic ionic end, hydrophilic
Structure of a Soap molecule
Cleansing Action of Soaps
Most dirt is oily in nature and the hydrophobic end attaches itself with dirt, while the ionic end is surrounded with molecules of water. This result in formation of a radial structure called micelles.
Long chain of carboxylic acid, hydrophobic ionic end, hydrophilic
Structure of a Soap molecule
Cleansing Action of Soaps
Most dirt is oily in nature and the hydrophobic end attaches itself with dirt, while the ionic end is surrounded with molecules of water. This result in formation of a radial structure called micelles.

- An emulsion is thus formed by soap molecule. The cloth needs to be mechanically agitated to remove the dirt particles from the cloth.
- Scum : The magnesium and calcium salts present in hard water reacts with soap molecule to form insoluble products called scum, thus obstructing the cleansing action. Use of detergents overcome this problem as the detergent molecule prevents the formation of insoluble product and thus clothes get cleaned.
Soaps and Detergents
- Soap is sodium and potassium salt of carboxylic acids with long chain.
- Soaps are effective with soft water only and ineffective with hard water.
- Detergents are ammonium or sulphonate salts of carboxylic acids with long chain. They are effective with both soft as well as hard water.
An ionic part (hydrophilic) and a long hydrocarbon chain (hydrophobic)part constitutes the soap molecule.

Long chain of carboxylic acid, hydrophobic ionic end, hydrophilic
Structure of a Soap molecule
Cleansing Action of Soaps
Most dirt is oily in nature and the hydrophobic end attaches itself with dirt, while the ionic end is surrounded with molecules of water. This result in formation of a radial structure called micelles.

- An emulsion is thus formed by soap molecule. The cloth needs to be mechanically agitated to remove the dirt particles from the cloth.
- Scum : The magnesium and calcium salts present in hard water reacts with soap molecule to form insoluble products called scum, thus obstructing the cleansing action. Use of detergents overcome this problem as the detergent molecule prevents the formation of insoluble product and thus clothes get cleaned.
Monday 17 September 2018
WORKSHEET
NUTRITION IN ANIMALS
CLASS VII
Question 2: Where is digested food absorbed in the body?
Question 3: Name the type of teeth which are for
Question 5: State whether the following statements are true or false
Question 7: How is food ingested by Amoeba?Explain with the help of a diagram
Question 8: What happens to food in stomach?
Question 9: Where is saliva produced? Write about its main functions.
Question 10: How is the process of digestion different in ruminants?
Question 11: What is a gall bladder? What is its role?
Question 12: What is villi ?where is it present?explain its role in digestion
Question 13: Why do we get instant energy from glucose?
Long answer type questions
Question 14: Briefly describe the process of nutrition in amoeba
Question 15: Write the functions of different types of teeth.
Question 16: Briefly explain the process of digestion in ruminants
Question 17: Draw a labeled diagram of human digestive system.
Question 18: Write about the various steps involved in the process of nutrition.
Answer to match the column type question
1 – b 2 – c 3 – a 4 – f 5 – d 6 – e 7 – j 8 – g 9 – h 10 –
NUTRITION IN ANIMALS
CLASS VII
Very short answer type questions
Question 1: Where is water from undigested food absorbed in the body?Question 2: Where is digested food absorbed in the body?
Question 3: Name the type of teeth which are for
- Chewing and grinding food
- Piercing and tearing food
- Biting and cutting food
Question 5: State whether the following statements are true or false
- The tongue helps in mixing saliva with food.
- Digestion of starch starts in stomach.
- Amino acids provide energy to our body.
- The blood takes digested food to all the cells of the body.
Short answer type questions
Question 6: What do you understand by term ‘assimilation’ in the process of digestion?Question 7: How is food ingested by Amoeba?Explain with the help of a diagram
Question 8: What happens to food in stomach?
Question 9: Where is saliva produced? Write about its main functions.
Question 10: How is the process of digestion different in ruminants?
Question 11: What is a gall bladder? What is its role?
Question 12: What is villi ?where is it present?explain its role in digestion
Question 13: Why do we get instant energy from glucose?
Long answer type questions
Question 15: Write the functions of different types of teeth.
Question 16: Briefly explain the process of digestion in ruminants
Question 17: Draw a labeled diagram of human digestive system.
Question 18: Write about the various steps involved in the process of nutrition.
Match the columns
| Column 1 | Column 2 |
| 1. frog | a. engulfs food by false feet |
| 2. hydra | b. catches insects with its tongue |
| 3. Amoeba | c. uses tentacles for ingestion of food |
| 4. spider | d. uses cilia for ingestion of food |
| 5. paramecium | e. uses proboscis to get food |
| 6. butterfly | f. lives on liquid food |
| 7. tearing of meat | g. milk teeth |
| 8. teeth that fall off in children | h. tongue |
| 9. taste buds | i. mastication |
| 10. chewing and mixing of food | j. canines |
1 – b 2 – c 3 – a 4 – f 5 – d 6 – e 7 – j 8 – g 9 – h 10 –
Subscribe to:
Posts (Atom)




































